Arctostaphylos uva-ursi (leaf)
Contents |
Nomenclature
Arctostaphylos uva-ursi (L.) Spreng. Ericaceae
Standardized common name (English): uva-ursi
Macroscopic Entries
|
Microscopic Entries
|
HPTLC Entries
|
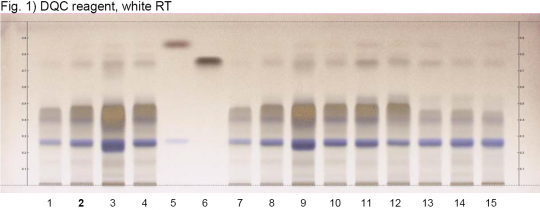
Uva-ursi (leaf) (Arctostaphylos uva-ursi) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 2.5 mg of arbutin in 1 mL of methanol. Dissolve 2.5 mg of hydroquinone in 1mL of methanol. Optional: Dissolve 2.5 mg of gallic acid in 1 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Ethyl acetate, formic acid, water 88:6:6 (v/v/v) Sample Preparation Method Sample: Mix 500 mg of powdered sample with 5 mL of methanol-water 1:1 and sonicate for 10 minutes at 60°C, then centrifuge or filter the solutations and use the supernatants / filtrates as test solutions. Derivatization reagent: 2,6-Dichloroquinone-4-chloroimide (DQC) reagent Preparation: 250 mg of DQC are dissolved in 50 mL of methanol. Use: Spray, dry in a stream of cold air for 3 min, expose to ammonia vapor (hold plate in a tank saturated with 32% ammonia) for 2 s or until blue zones are visible. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Arbutin: blue zone at Rf ~ 0.27. Hydroquinone: brown zone at Rf ~ 0.87. Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows an intense blue zone at Rf ~ 0.27 corresponding to reference substance arbutin. Above it there is another blue zone Rf ~ 0.40 and overlapping a very broad and diffuse brown zone. A faint brown zone is seen at Rf ~ 0.75 (gallic acid). Some samples show a faint brown zone at Rf ~ 0.87 (hydroquinone).
|
Other Points of Interest
Cite error: <ref> tags exist, but no <references/> tag was found