Cichorium intybus (root)
(→USD) |
(Nomenclature updated) |
||
| Line 1: | Line 1: | ||
| − | = | + | =Nomenclature= |
| + | |||
| + | {{nomenclature | binomial=Cichorium intybus | ||
| + | |authority=L. | ||
| + | |family=Asteraceae | ||
| + | |scn=chicory | ||
| + | |syn= | ||
| + | |ayurvedic=kasni | ||
| + | |pinyin=ju ju (aboveground parts) | ||
| + | |aka= | ||
| + | |notes= }} | ||
| + | |||
=Macroscopic Entries= | =Macroscopic Entries= | ||
{{Macroscopy | source=United States Dispensatory (1918) | {{Macroscopy | source=United States Dispensatory (1918) | ||
Revision as of 22:17, 13 March 2014
Contents |
Nomenclature
Cichorium intybus L. Asteraceae
Standardized common name (English): chicory
Ayurvedic name(s): kasni
Pinyin name(s): ju ju (aboveground parts)
Macroscopic Entries
|
Microscopic Entries
|
HPTLC Entries
|
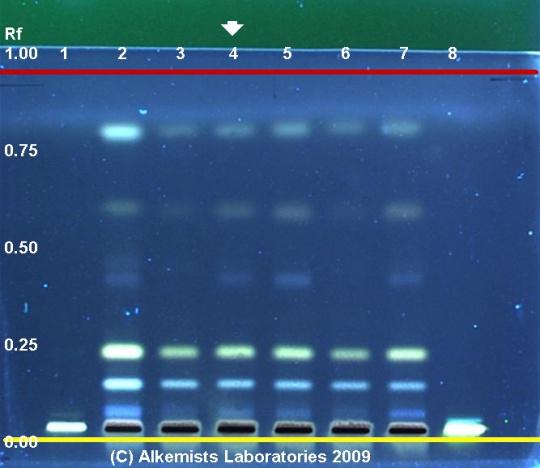
Chicory (root) (Cichorium intybus) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference materials used here have been authenticated by macroscopic, microscopic &/or TLC studies according to the reference source cited below held at Alkemists Pharmaceuticals, Costa Mesa, CA. Stationary Phase Silica gel 60, F254, 10 x 10 cm HPTLC plates Mobile Phase CH2Cl2: Petroleum ether [8/2] Sample Preparation Method 0.3 g + 3 ml CH3OH sonicated + heated @ 50° C ~ 1 hr. Detection Method 10% Ethanolic H2SO4 -> 115° C 15 min -> UV 365 nm Reference see Pharmacopoeia of The Peoples Republic of China, Volume 1, 2005
|
Other Points of Interest
Cite error: <ref> tags exist, but no <references/> tag was found