Lavandula latifolia (flower)
(Nomenclature updated) |
(Various title corrections, Source additions) |
||
| Line 1: | Line 1: | ||
| + | {{DISPLAYTITLE:''Lavandula latifolia'' (flower) }} | ||
=Nomenclature= | =Nomenclature= | ||
| Line 11: | Line 12: | ||
|notes= }} | |notes= }} | ||
| + | =Botanical Voucher Specimen= | ||
| + | =Organoleptic Characteristics= | ||
| − | =Macroscopic | + | =Macroscopic Characteristics= |
| − | + | =Microscopic Characteristics= | |
| − | = | + | =High Performance Thin Layer Chromatographic Identification= |
| − | + | ||
| − | + | ||
| − | + | ||
{{HPTLC | source=HPTLC Association | {{HPTLC | source=HPTLC Association | ||
| companyimage=HPTLC-assoc-Logo-farbig-Text-schwarz-300x47.png | | companyimage=HPTLC-assoc-Logo-farbig-Text-schwarz-300x47.png | ||
| Line 62: | Line 62: | ||
| − | = | + | =Supplementary Information= |
| − | + | =Sources= | |
| − | + | <references /> | |
Revision as of 01:17, 16 March 2014
Contents |
Nomenclature
Lavandula latifolia Medic. Lamiaceae
Standardized common name (English): spike lavender
Botanical Voucher Specimen
Organoleptic Characteristics
Macroscopic Characteristics
Microscopic Characteristics
High Performance Thin Layer Chromatographic Identification
|
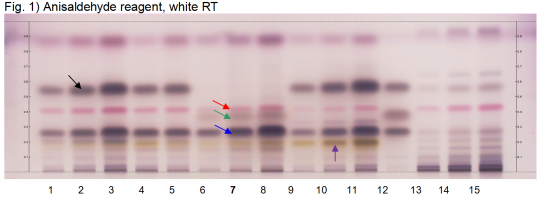
Spike lavender oil (flower) (Lavandula latifolia) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 5 µL of linalool in 1 mL of toluene; Dissolve 5 µL of linalyl acetate in 1mL of toluene; Optional: Dissolve 10 µL of cineole in 1 mL of toluene. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Toluene, ethyl acetate 95:5 (v/v) Sample Preparation Method Sample:Mix 500 mg of powdered sample with 5 mL of toluene and sonicate for 10 minutes, centrifuge or filter the solutions and use the supernatants / filtrates as test solutions. Derivatization reagent: Anisaldehyde reagent, Preparation: 170 mL of ice-cooled methanol are mixed with 20 mL of acetic acid, 10 mL of sulfuric acid and 1 mL of anisaldehyde, Use: Dip (time 0, speed 5), heat at 100°C for 5 min. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Linalyl acetate: violet zone at Rf ~ 0.57; Linalool: violet zone at Rf ~ 0.27. Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows a violet zone at Rf ~ 0.27 corresponding to reference linalool (blue arrow) and a brownish zone due to cineole (green arrow). Above this zone there is a pink zone at Rf ~ 0.42 (red arrow). A faint violet zone at Rf ~ 0.57 corresponding to linalyl acetate may be present. Test for other species: The chromatograms of Lavender oil and Lavanin show an intense violet zone at the position of linalyl acetate (black arrow). Lavender flower shows no yellow zone below the linalool reference (violet arrow).
|
Supplementary Information
Sources
- ↑ HPTLC Association http://www.hptlc-association.org/