Terminalia chebula (fruit)
(Nomenclature updated) |
(Various title corrections, Source additions) |
||
| Line 1: | Line 1: | ||
| + | {{DISPLAYTITLE:''Terminalia chebula'' (fruit) }} | ||
=Nomenclature= | =Nomenclature= | ||
| Line 11: | Line 12: | ||
|notes= }} | |notes= }} | ||
| − | = | + | =Botanical Voucher Specimen= |
| − | + | =Organoleptic Characteristics= | |
| − | = | + | =Macroscopic Characteristics= |
| − | + | =Microscopic Characteristics= | |
| − | = | + | =High Performance Thin Layer Chromatographic Identification= |
{{HPTLC | source=HPTLC Association | {{HPTLC | source=HPTLC Association | ||
| companyimage=HPTLC-assoc-Logo-farbig-Text-schwarz-300x47.png | | companyimage=HPTLC-assoc-Logo-farbig-Text-schwarz-300x47.png | ||
| Line 98: | Line 99: | ||
| − | = | + | =Supplementary Information= |
| − | + | =Sources= | |
| − | + | <references /> | |
Revision as of 02:47, 16 March 2014
Contents |
Nomenclature
Terminalia chebula Retz. Combretaceae
Standardized common name (English): chebulic myrobalan
Ayurvedic name(s): haritaki
Pinyin name(s): he zi (fruit)
Botanical Voucher Specimen
Organoleptic Characteristics
Macroscopic Characteristics
Microscopic Characteristics
High Performance Thin Layer Chromatographic Identification
|
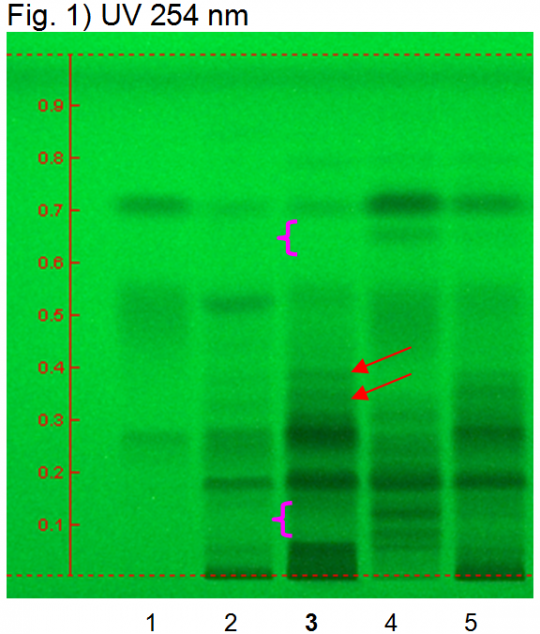
Chebulic myrobalan fruit, he zi (fruit) (Terminalia chebula) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
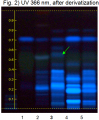
Reference Sample(s) Reference: Dissolve 1 mg of chebulinic acid and 2 mg gallic acid in 10 mL of methanol. Optional: dissolve 1 mg of ellagic acid in 10 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Ethyl formate, toluene, formic acid, water 30:1.5:4:3 (v/v/v/v) Sample Preparation Method Sample: Mix 1.0 g of powdered sample with 10 mL of methanol and sonicate for 10 minutes, then centrifuge or filter and use the supernatants/filtrates as test solutions. Derivatization reagent: 1.) NP reagent, Preparation: 1 g of natural products reagent in 200 mL of ethyl acetate; 2.) PEG reagent, Preparation: 10 g of polyethylene glycol 400 in 200 mL of dichloromethane, Use: Heat plate for 3 min at 100°C, dip (time 0, speed 5) in NP reagent, dry, dip (time 0, speed 5) in PEG reagent Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Chebulinic acid: a quenching zone at Rf ~ 0.26 (UV 245 nm). Gallic acid: a quenching zone at Rf ~ 0.71(UV 245 nm). Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. Under UV 254 nm the test solution shows three intense quenching zones at Rf ~ 0.05, 0.19 and 0.28. A characteristic quenching double zone is present at Rf ~ 0.40/0.38 (red arrows). At the position of gallic acid a quenching zone is visible. Under UV 366 nm there are blue fluorescent zones at Rf ~ 0.23 and Rf ~ 0.38 and a characteristic fluorescent zone at Rf ~ 0.53 (green arrow). Test for other species: Under UV 254 nm there is no zone between Rf ~ 0.08 and 0.12 and no zone at Rf ~ 0.66 (Amla fruit, pink arrows). Under UV 366 nm no fluorescent zone is detectable at Rf ~ 0.52 (Belleric myrobalan fruit). Source: HPTLC Association [1] |
|
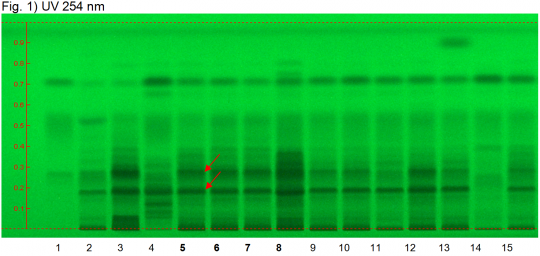
Triphala powder (mixture of Phyllanthus emblica, Terminalia bellerica, Terminalia chebula) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 1 mg of chebulinic acid and 2 mg of gallic acid in 10 mL of methanol. Optional: dissolve 1 mg of ellagic acid in 10 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Ethyl formate, toluene, formic acid, water 30:1.5:4:3 (v/v/v/v) Sample Preparation Method Sample: Mix 1.0 g of powdered sample with 10 mL of methanol and sonicate for 10 minutes, then centrifuge or filter and use the supernatants/filtrates as test solutions. Derivatization reagent: 1.) NP reagent, Preparation: 1 g of NP reagent in 200 mL of ethyl acetate; 2.) PEG reagent, Preparation: 10 g of polyethylene glycol 400 in 200 mL of dichloromethane, Use: Heat plate 3min at 100°C, dip (time 0, speed 5) in NP reagent, dry and dip (time 0, speed 5) in PEG reagent. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Chebulinic acid: a quenching zone at Rf ~ 0.26 (UV 245 nm). Gallic acid: a quenching zone at Rf ~ 0.71 (UV 245 nm). Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. Under UV 254 nm the test solution shows several diffuse quenching zones above the application position. The two main quenching zones are detected at Rf ~ 0.19 and Rf ~ 0.28 (red arrows). Above these zones there are additional diffuse quenching zones. There are quenching zones at the position of ellagic acid and of gallic acid. Under UV 366 nm two blue fluorescent zones are detected right above the application position (yellow arrow). Several intense blue fluorescent zones are present between Rf ~ 0.16 and 0.40. At the position of gallic acid there is a fluorescent zone. Source: HPTLC Association [2] |
Supplementary Information
Sources
- ↑ HPTLC Association http://www.hptlc-association.org/
- ↑ HPTLC Association http://www.hptlc-association.org/