Curcuma longa (rhizome)
Nomenclature
Curcuma longa L. Zingiberaceae
Syn. Curcuma domestica Valeton
Standardized common name (English): turmeric
Ayurvedic name(s): haridra
Pinyin name(s): jiang huang; jiang huang (rhizome); yu jin (root tuber)
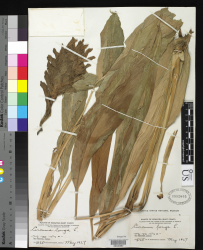
Botanical Voucher Specimen
 |
|
|
Organoleptic Characteristics
|
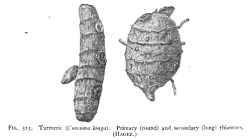
Macroscopic Characteristics
|
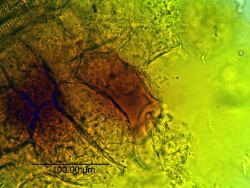
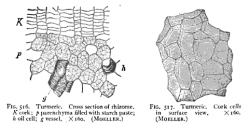
Microscopic Characteristics
|
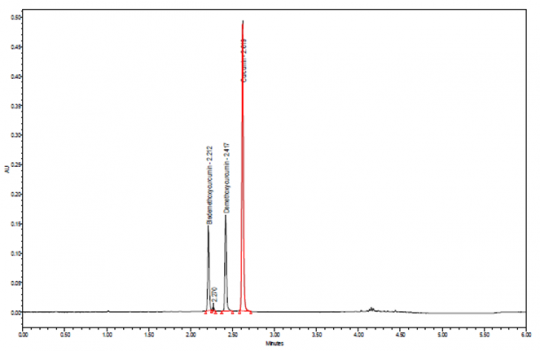
Ultra Performance Liquid Chromatographic Identification
|
Method Test Sample Preparation: 1 g of ground plant material into a Soxhlet thimble and extractovernight with about 90 ml of acetone, centrifuge and use the supernatant. Dilute the extract 1:10 in acetone and mix well. Before injection, filter through a 0.20 um PTFE membrane filter. Column: 100-mm x 2.1-mm, 1.7 um, Waters Acquity BEH C18 Mobile Phase: 0.1% formic acid in water (Solution A) and acetonitrile (Solution B) Elution: Gradient, see Table below Column Temperature: 70°C Flow rate: 0.6 mL/min Detection: Vis, 426 nm Injection volume: 1.0 uL, maintained at 10°C Needle wash: Acetonitrile System suitability: Curcumin peak, tailing 0.8 to 1.5 Source: Indena S.p.A. [12] |
Table: Gradient program
| Time (min) | Solution A (%) | Solution B (%) |
| 0.00-3.20 | 70-47 | 30-53 |
| 3.20-4.10 | 47-5 | 53-95 |
| 4.10-5.10 | 5 | 95 |
| 5.10-5.15 | 5-70 | 95-30 |
| 5.15-6.00 | 70 | 30 |
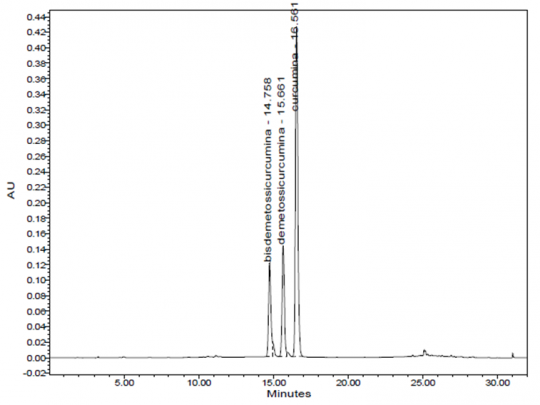
High Performance Liquid Chromatographic Identification
|
Method Test Sample Preparation: 1 g of ground plant material into a Soxhlet thimble and extract overnight with about 90 ml of acetone, centrifuge and use the supernatant. Dilute the extract 1:10 in acetone and mix well. Before injection, filter through a 0.20 um PTFE membrane filter. Column: 25-cm x 4.6-mm, 5 um, Waters Symmetry C18 Mobile Phase: 0.3% acetic acid in water (Solution A) and acetonitrile (Solution B) Elution: Gradient, see Table below Column Temperature: 35°C Flow rate: 1.0 mL/min Detection: Vis, 426 nm Injection volume: 10 uL System suitability: Curcumin peak, tailing 0.8 to 2.0; resolution between demethoxycurcumin and curcumin is ≥ 2.0 Source: Indena S.p.A. [13] |
Table: Gradient program
| Time (min) | Solution A (%) | Solution B (%) |
| 0-17 | 60-40 | 40-60 |
| 17-18 | 40-0.0 | 60-100 |
| 18-24 | 0.0 | 100 |
| 24-25 | 0.0-60 | 100-40 |
| 25-32 | 60 | 40 |
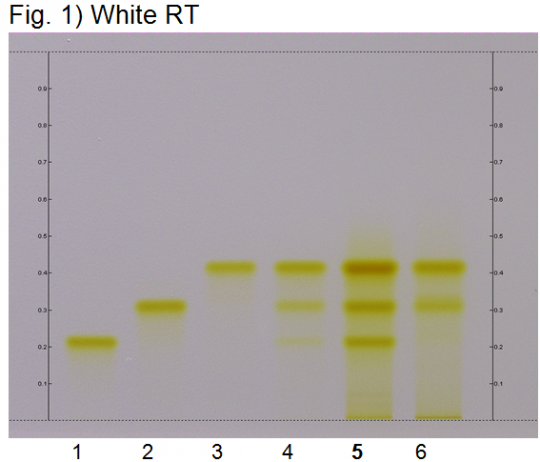
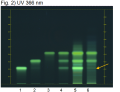
High Performance Thin Layer Chromatographic Identification
|
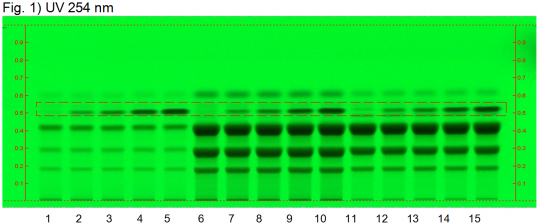
Turmeric (rhizome) (Curcuma longa) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 2 mg of USP Curcuminoids RS in 5 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Toluene, acetic acid (4:1) (v/v) Sample Preparation Method Sample: Mix 0.2 g of powdered sample with 3 mL of methanol, sonicate for 10 minutes, then centrifuge or filter the solution and use the supernatant/filtrate as test solution. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test (UV 366 nm): Curcuminoids: three yellowish-green zones at Rf ~ 0.21 (bisdesmethoxycurcumin), Rf ~ 0.32 (desmethoxycurcumin), and Rf ~ 0.42 (curcumin). Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows three yellowish-green zones at Rf ~ 0.21, Rf ~ 0.32, and Rf ~ 0.42 corresponding to the three zones of the curcuminoids reference. Test for other species: The chromatogram of Curcuma xanthorriza does not show an intense fluorescent zone at Rf ~ 0.21 (orange arrow). Source: HPTLC Association [14] |
Supplementary Information
Detection of adulteration of products of Curcuma longa rhizome with the anti-inflammatory drug nimesulide
|
Turmeric adulteration with nimesulide (rhizome) (Curcuma longa) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 1.0 mg of nimesulide in 10 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Toluene, acetic acid (4:1) (v/v) Sample Preparation Method Sample: Mix 0.2 g of powdered sample with 3 mL of methanol, sonicate for 10 minutes, then centrifuge or filter the solution and use the supernatant/filtrate as test solution. Derivatization reagent: 2,5-Dichloro-1,4-benzoquinone reagent, Preparation: 0.50 g of 2,5-Dichloro-1,4-benzoquinone is dissolved in 80 mL of dimethyl sulfoxide and then diluted with 160 mL of tetrahydrofuran, Use: Dip (time 0, speed 5), dry in a stream of cold air for 3 min. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Nimesulide: Rf ~ 0.51 Test for adulteration: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows no zone at Rf ~ 0.51 corresponding in color and position to that of the nimesulide reference standard. Source: HPTLC Association [15] |
Sources
- ↑ Encyclopedia of Life (Smithsonian Institution, National Museum of Natural History, Department of Botany) http://eol.org/data_objects/25071286
- ↑ Winton, A. (1916) Microscopy of vegetable foods, 2nd ed.
- ↑ Winton, A. (1916) Microscopy of vegetable foods, 2nd ed.
- ↑ PlantaPhile http://plantaphile.com/
- ↑ PlantaPhile http://plantaphile.com/
- ↑ Winton, A. (1916) Microscopy of vegetable foods, 2nd ed.
- ↑ Culbreth, D. (1917) A Manual of Materia Media and Pharmacology, 6th ed.
- ↑ Winton, A. (1916) Microscopy of vegetable foods, 2nd ed.
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ Winton, A. (1916) Microscopy of vegetable foods, 2nd ed.
- ↑ Indena S.p.A. http://www.indena.com/
- ↑ Indena S.p.A. http://www.indena.com/
- ↑ HPTLC Association http://www.hptlc-association.org/
- ↑ HPTLC Association http://www.hptlc-association.org/
- Botanical
- Zingiberaceae
- Media
- Voucher
- Encyclopedia of Life (Smithsonian Institution, National Museum of Natural History, Department of Botany)
- Macroscopy
- Winton, A. (1916) Microscopy of vegetable foods, 2nd ed.
- PlantaPhile
- Microscopy
- Culbreth, D. (1917) A Manual of Materia Media and Pharmacology, 6th ed.
- Elan M. Sudberg, Alkemist Laboratories
- Indena S.p.A.
- HPTLC
- HPTLC Association