Hoodia gordonii (aerial parts)
Contents |
Nomenclature
Botanical Voucher Specimen
 |
 |
 |
 |
|
|
|
|
|
 |
 |
|
|
|
Organoleptic Characteristics
Macroscopic Characteristics
Microscopic Characteristics
High Performance Thin Layer Chromatographic Identification
|
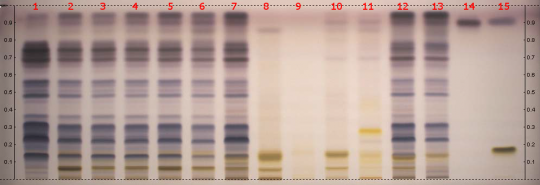
Hoodia (aerial parts) (Hoodia gordonii) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Samples for tracks 1–11 were provided by CAMAG. Tracks 12 and 13 were made samples of Hoodia gordonii material obtained by AHPA from South Africa, the identity of which were verified by Ernst van Jaarsveld, curator of Conservatory in Kirstenbosch Gardens in Capetown. The material for track 13 was treated for preservation purposes. Reference Sample(s) Dissolve 7 mg of fructose in 5 mL of ethanol/water 7:3 Dissolve 2 mg of beta-sitosterol in 5 mL of methanol Dissolve 0.6 mg of P57 in 2 mL of methanol Stationary Phase Plate: Merck HPTLC glass 20x10 cm, Si 60 F254 batch HX 745979 Mobile Phase chloroform/methanol/water 70:30:3 Sample Preparation Method Sample: Mix 0.5 g of powdered sample with 5 mL methanol or methanol/water 8:2, then sonicate for 10 min and centrifuge. Standard: Dissolve 7 mg of fructose in 5 mL of ethanol/water 7:3 Dissolve 2 mg of beta-sitosterol in 5 mL of methanol Dissolve 0.6 mg of P57 in 2 mL of methanol Plate: Apply 5 μL of the prepared samples and standards as 8 mm wide bands, which should be at least 2 mm apart and 8 mm from the lower edge of the plate. Detection Method Derivitizing agent: anisaldehyde Preparation: 10 mL sulfuric acid is carefully added to an ice-cooled mixture of 170 mL methanol and 20 mL acetic acid, before adding 1 mL anisaldehyde Application: the plate is dipped then heated at 100°C for 3 min Other Notes Developing distance from application position/lower edge: 62/70 mm Developing solvent: chloroform/methanol/water 70:30:3 Developing time: 10 min Plate drying: 5 min in a stream of cold air Equipment: CAMAG Automatic TLC Sampler 4, CAMAG Automatic Developing Chamber (ADC2) with humidity control (MgCl2 to RH 33%), CAMAG Chromatogram Immersion Device III, CAMAG TLC Plate Heater III, CAMAG Digistore 2, and CAMAG filter paper for chamber saturation base ADC2, IKA Mill KB5/10, Hettich Centrifuge EBA21, Telsonic Ultrasonic Bath TPC25, and Mettler-Toledo DC4400 and AG245 Balances. Further methodology available here, in AHPA Resource HPTLC of Hoodia gordonii.
|
Supplementary Information
Hoodia (aerial parts, powder) (Hoodia gordonii)
General Characteristics AHPA recommends in its Known Adulterants list that appropriate steps be taken to assure that this raw material is free of the noted adulterant. Contact AHPA for additional information regarding relevant analytical methods or follow this link for more information.
Reported Adulterants Various powders possibly including Opuntia spp. and other Hoodia species.
Source: AHPA Known Adulterants [8]
AHPA Practical: Identification and Purity Determination of Hoodia gordonii Powdered Stem
Introduction
The American Herbal Products Association is providing here analytical tools for identification and determination of purity of powdered raw materials labeled as Hoodia gordonii stems. The work was commissioned by the AHPA Hoodia gordonii Committee and managed by AHPA staff. It was initiated through solicitation of proposals that were evaluated by an independent expert scientific review panel.
The tools consist of three methods for the characterization and identification of material labeled as Hoodia gordonii stem. These are microscopy, high-performance thin layer chromatography (HPTLC), and high-performance liquid chromatography (HPLC). These techniques are complementary tools to provide information regarding identity and purity of raw powdered material, and should be employed in concert.
Microscopy
When employed by a qualified microscopist trained in analyzing botanical powders, this technique is capable of revealing the presence of cell components that are unique to a test material as well as negative markers (i.e., cell structures that do not occur in a test material).
The images provided here show characteristics of authentic material, but microscopic features indicative only of Hoodia gordonii stem have not yet been found. So far microscopy cannot reliably differentiate Hoodia gordonii from related Hoodia species or from Caralluma fimbriata. However, the presence of calcium oxalate crystals, and specific cellular substructures known as sclereids and stone cells always indicate the presence of material that is NOT from Hoodia species.
While not capable of positively identifying Hoodia gordonii stem by itself, microscopy is nevertheless very useful for detecting certain added adulterants. Microscopy is able to reveal the presence of Opuntia spp. added to Hoodia gordonii powder at a 1% concentration. Similarly the presence of maltodextrin can be detected reliably at 10% adulteration and possibly even at 1% and 5% concentrations.
- Click here for Microscopy images and a technique to detect adulteration with maltodextrin or Opuntia spp.
Note: Not all web browsers present this linked page properly. Internet Explorer is among those that does show the page.
HPTLC
When properly employed, High Performance Thin Layer Chromatography (HPTLC) provides a visual display of compounds present in test materials. However, this does not necessarily mean that HPTLC will always provide authentication to the exclusion of related species and all possible adulterants.
The HPTLC method used here is a very powerful tool for identification of the presence of Hoodia gordonii stem based on the characteristic image produced. The extent of variability of results for authentic materials has not yet been determined, nor has the extent of HPTLC to detect mixtures of authentic material with added adulterants.
- Click here for an HPTLC method for Hoodia gordonii.
HPLC
Recent advances in chromatography has made the High Performance Liquid Chromatography (HPLC) very useful for the analysis of targeted analytes in complex matrices such as botanical-based dietary supplements. The rigour and confidence in an analytical result is determined by a number of key factors including the competence of the analyst, the method selection and appropriateness, reference materials employed, and finally the test material itself. Two main approaches may be employed for HPLC analysis. They are marker or active determination, and profiling (often referred to as “fingerprinting”) techniques.
While it may sometimes be preferable to quantify a specified constituent or class of compounds, the selection of analytes and methods of analysis can be driven by marketing, ease of analysis, or other relatively arbitrary reasons. It is very important when selecting or developing an analytical method to carefully consider, “Will the question being asked be answered by the analysis results?” Always use analytical methods within their specified scope and applicability. For Hoodia gordonii the question was simply, “Is this material Hoodia gordonii or not?”
The question of identity is actually the hardest one to answer by any analytical technique. To definitively answer it there must be diagnostic features such as morphological, chemical, or genetic that can be used to differentiate Hoodia gordonii from related species and known adulterants. Certain identified marker compounds known as oxypregnane glycosides have been employed to determine the presence of Hoodia species. Although the best known of these, P57, has been used as a marker for Hoodia gordonii it is not necessarily diagnostic for this species alone because P57 can be found in other Hoodia species. Furthermore, published HPLC methods of analysis for P57 can be confounded by co-eluting peaks that are found in adulterating species. For example the mistaken assignment of P57 in Gymmema sylvestre can occur if the HPLC conditions are not optimized for this problem as demonstrated in Figure 1.
- Click here for an HPLC method for Hoodia gordonii.
A recent publication by Avula et al. reported similar findings. Lengthening the HPLC run time for this method (see Figure 2) now separates the peak in Gymnema sylvestre, as shown in Figure 3, which previously corresponded to that of P57 with the unmodified method. The better optimized method clearly reveals 11 oxypregnane glycosides from Hoodia gordonii as shown in Figure 4. Figure 5 demonstrates the wide variety of HPLC “fingerprints” that can be generated for Hoodia gordonii. Future work may entail the acquisition of additional samples so that a wider chemical representation of Hoodia gordonii materials can be subjected to this evaluation. Discrimination from other potentially adulterating species, such as Stapelia spp., will be aided by their evaluation with this method and comparison of the results.
Acknowledgements
- The microscopy work was done by AHPA member company Alkemists Pharmaceuticals, Inc. under the direction of Elan Sudberg.
- The HPTLC work was done by AHPA member company CAMAG Scientific, Inc. under the direction of Eike Reich, PhD.
- The HPLC work was done by Flora Research Laboratories under the direction of James Neal-Kababick. This was a joint effort of Flora Research Laboratories and the NHP Research Group at the British Columbia Institute of Technology (BCIT) with thanks to Dr. Ikhlas Kahn at University of Mississippi School of Pharmacy for providing standards and information on his HPLC separation methods to optimize the method.
In addition, this project was funded by numerous companies and individuals, including:
- Desert Burn Industries, LLC
- Ethno Africa, LLC
- Herbal Teas International
- Millennium Health
- Nature’s Way Products, Inc.
- NOW Foods
- Vitality Works
- Wulff Africeuticals, a division of WulffCapital, LP
Sources
- ↑ Royal Botanic Gardens, Kew. http://specimens.kew.org/herbarium/K000306192
- ↑ Royal Botanic Gardens, Kew. http://specimens.kew.org/herbarium/K000306196
- ↑ Royal Botanic Gardens, Kew. http://specimens.kew.org/herbarium/K000306197
- ↑ Royal Botanic Gardens, Kew. http://specimens.kew.org/herbarium/K000306198
- ↑ Royal Botanic Gardens, Kew. http://specimens.kew.org/herbarium/K000306198
- ↑ Royal Botanic Gardens, Kew. http://specimens.kew.org/herbarium/K000306202
- ↑ AHPA Practical, CAMAG HPTLC http://www.camag.com/
- ↑ AHPA Known Adulterants http://www.ahpa.org/